A team of researchers, which included scientists from SLAC, Stanford, the University of California, Berkeley and DOEâs Lawrence Berkeley National Laboratory, recently explained a process that interferes with making quantum dots brighter - when attempting to increase the intensity of emitted light, heat is generated instead - reducing the dotsâ light-producing efficiency. The results of this new work could have broad implications for developing future quantum and photonics technologies.
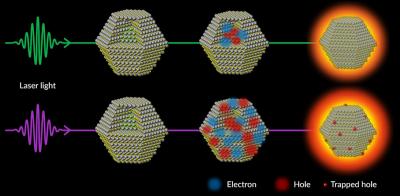 Image credit: Nature Communications/SLAC
Image credit: Nature Communications/SLAC
In a QLED TV screen, dots absorb blue light and turn it into green or red. At the low energies where TV screens operate, this conversion of light from one color to another is virtually 100% efficient. But at the higher excitation energies required for brighter screens and other technologies, the efficiency drops sharply. Researchers theorized about why this happens, but no one had ever observed it at the atomic scale until now.
The experiments revealed that the incoming high-energy laser light ejects electrons from the dotâs atoms, and their corresponding holes â empty spots with positive charges that are free to move around â become trapped at the surface of the dot, producing unwanted waste heat.
In addition, electrons and holes recombine in a way that gives off additional heat energy. This increases the jiggling of the dotâs atoms, deforms its crystal structure and wastes even more energy that could have gone into making the dots brighter.
âThis represents a key way that energy is sucked out of the system without giving rise to light,â said Aaron Lindenberg, a Stanford University associate professor and investigator with the Stanford Institute for Materials and Energy Sciences at SLAC who led the study with postdoctoral researcher Burak Guzelturk.
"Trying to figure out what underlies this process has been the subject of study for decades,â he said. âThis is the first time we could see what the atoms are actually doing while excited state energy is being lost as heat.â
Despite their tiny size, quantum dot nanocrystals are surprisingly complex and highly engineered. They emit extremely pure light whose color can be tuned by adjusting their size, shape, composition and surface chemistry. The quantum dots used in this study were invented more than two decades ago, and today theyâre widely used in bright, energy-efficient displays and in imaging tools for biology and medicine.
Previous studies had focused on how the dotsâ electrons behaved. But in this study, the team was able to see the movements of whole atoms, too, with an electron camera known as MeV-UED. It hits samples with short pulses of electrons with very high energies, measured in millions of electronvolts (MeV). In a process called ultrafast electron diffraction (UED), the electrons scatter off the sample and into detectors, creating patterns that reveal what both electrons and atoms are doing.
As the SLAC/Stanford team measured the behavior of quantum dots that had been hit with various wavelengths and intensities of laser light, UC Berkeley graduate students Dipti Jasrasaria and John Philbin worked with Berkeley theoretical chemist Eran Rabani to calculate and understand the resulting interplay of electronic and atomic motions from a theoretical standpoint.
âWe met with the experimenters quite often,â Rabani said. âThey came with a problem and we started to work together to understand it. Thoughts were going back and forth, but it was all seeded from the experiments, which were a big breakthrough in being able to measure what happens to the quantum dotsâ atomic lattice when itâs intensely excited.â
The study was carried out by researchers in a DOE Energy Frontier Research Center, Photonics at Thermodynamic Limits, led by Jennifer Dionne, a Stanford associate professor of materials science and engineering and senior associate vice provost of research platforms/shared facilities. Her research group worked with Lindenbergâs group to help develop the experimental technique for probing the nanocrystals.
"To create photonic thermodynamic cycles, you need to precisely control how light, heat, atoms, and electrons interact in materials,â Dionne said. âThis work is exciting because it provides an unprecedented lens on the electronic and thermal processes that limit the light emission efficiency. The particles studied already have record quantum yields, but now there is a path toward designing almost-perfect optical materials.â Such high light emission efficiencies could open a host of big futuristic applications, all driven by tiny dots probed with ultrafast electrons.